Page 3 - reflections_dyslipidaemia_newsletter7_2024
P. 3
REFLECTIONS
Dyslipidaemia
Dyslipidaemia Global Newsletter #7 2024
TREATMENT Dyslipidaemia
Is there a role for earlier use of combination therapy?
Revankar S, et al. Am J Prev Cardiol. 2024 Feb 15:17:100639.
Despite the adoption of statins as the cornerstone of lipid therapy regimens, many high-risk patients need combination therapy
with ezetimibe to achieve LDL-C goals. Combination therapy of statin plus ezetimibe has been shown to decrease LDL-C levels,
atheroma volume, and CV adverse event rates. Despite this, many clinical guidelines continue to recommend combination therapy in
only very high-risk patients, with a stepwise intensification approach in other CV risk groups.
The authors provide a review of the evidence to support not only the original combination therapy of statins and ezetimibe, but
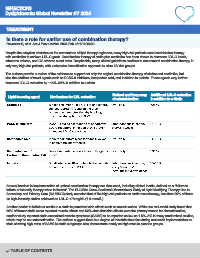
also the addition of novel agents such as PCSK-9 inhibitors, bempedoic acid, and inclisiran to a statin. These agents may further
decrease LDL-C reduction by ~14%–62% in addition to a statin.
Method and frequency Additional LDL-C reduction
Lipid-lowering agent Mechanism for LDL reduction of administration in addition to a Statin
Several barriers to implementation of upfront combination therapy are discussed, including clinical inertia, defined as a “failure to
initiate or intensify therapy when indicated”. The EU-Wide Cross-Sectional Observational Study of Lipid Modifying Therapy Use in
Secondary and Primary Care (DA VINCI) study revealed that of the high-risk patients on statin monotherapy, less than 30% of those
on high-intensity statins achieved an LDL-C <70 mg/dL (1.8 mmol/L).
Another barrier to initiate or continue a statin is perceived side effects such as muscle aches. While one real-world study found that
60% of former statin users reported muscle effects and 62% state that side effects were the primary reason for discontinuation,
another study reported statin-associated muscle symptoms (SAMS) to be reported as low as 1.5%–5% in many randomized studies,
which may be an underestimation. The authors suggest there is a degree of misattribution bias during real-world implementation as
trials showing high rates of SAMS in statin subgroups also demonstrate nearly as high rates in placebo groups.
TABLE OF CONTENTS